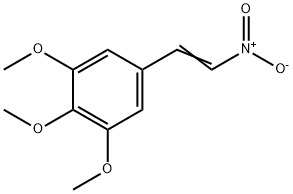
1-(3,4,5-TRIMETHOXYPHENYL)-2-NITROETHENE(CAS#6316-70-7)
Core chemical properties
Functional group reaction characteristics:
Carbon carbon double bond activity: The molecule contains conjugated carbon carbon double bonds (- CH=CH -), which combine the stability of aromatic compounds with the reactivity of olefins. They are prone to Michael addition, Diels Alder cycloaddition, and other reactions, making them key sites for constructing complex cyclic or chain structures;
Characteristics of nitro group (- NO ₂): nitro group is a strong electron withdrawing group, which reduces the electron cloud density of conjugated double bonds, enhances the electrophilicity of double bonds, and facilitates addition reactions with nucleophilic reagents such as amines and thiols; Nitro groups can be converted to amino groups (- NH ₂) under reducing conditions such as palladium carbon catalytic hydrogenation and iron powder/hydrochloric acid reduction, providing a basis for subsequent functional group conversion.
Typical chemical reaction:
Reduction reaction: Nitro (- NO ₂) can be selectively reduced to amino, generating 1- (3,4,5-trimethoxyphenyl) -2-aminoethylene, which is the core step in the preparation of aromatic amine intermediates;
Addition reaction: nucleophilic addition reaction with amine compounds to construct C-N bonds, used for synthesizing nitrogen-containing heterocycles or amine derivatives;
Stability: It is not easily oxidized at room temperature and does not react significantly with common oxidants such as potassium permanganate and hydrogen peroxide under neutral conditions; Avoid long-term contact with strong reducing agents and strong bases (such as potassium tert butoxide), which may cause double bond isomerization or nitro reduction side reactions.
Other chemical properties:
No obvious hygroscopicity: Unlike potassium tert butoxide, this product has good stability in air and no significant hygroscopicity or deliquescence;
Non explosive: The solid itself is not flammable, with a flash point greater than 150 ℃, and does not belong to the category of flammable solids or liquids in hazardous chemical classification;
Low corrosiveness: It has no significant corrosiveness to metals such as iron, aluminum, and stainless steel, and can be stored in conventional glass or plastic containers.
4、 Core application areas
As an aromatic intermediate containing multiple functional groups, its applications are concentrated in the fields of medicine, pesticides, and fine chemicals, with core uses including:
Synthesis of drug intermediates: Used for the preparation of active ingredients such as antimalarials, anti-inflammatory drugs, anti-tumor drugs, etc., especially in the synthesis of heterocyclic drugs (such as quinoline and pyrrole), the core skeleton of drug molecules is constructed through Michael addition and cyclization reactions;
Preparation of pesticide active ingredients: used as key intermediates for synthesizing fungicides and herbicides, utilizing the reactivity of conjugated double bonds with heteroatoms to introduce functional groups with biological activity;
Fine chemical synthesis: As a “building block” reagent in organic synthesis, it is used to prepare functional monomers for special dyes, fragrances, and polymer materials. Its trimethoxyphenyl structure can enhance the stability and biocompatibility of the products.