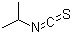Phenylethyl isothiocyanate(CAS#2257-09-2)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. R42 – May cause sensitization by inhalation R42/43 – May cause sensitization by inhalation and skin contact. |
| Safety Description | S23 – Do not breathe vapour. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S36/37 – Wear suitable protective clothing and gloves. |
| UN IDs | UN 2206 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | NX9115000 |
| FLUKA BRAND F CODES | 10-23 |
| TSCA | Yes |
| HS Code | 29309090 |
| Hazard Class | 8 |
| Packing Group | III |
| Toxicity | LD50 in mice (mg/kg): 700 orally; 150 s.c.; 50 i.v. (Lichtenstein) |
Introduction
Phenethyl isothiocyanate is an organic compound. 2. Solubility: It can dissolve in organic solvents such as alcohols, ethers, and esters, but is insoluble in water. 3. Stability: It is relatively stable at room temperature, but can decompose when exposed to heat, light, or reactions with oxygen or acids.The main uses of phenethyl isothiocyanate are as follows:1. Chemical synthesis: It can be used as a reagent in organic synthesis, functioning as a catalyst, protecting group, or activator in various chemical reactions.2. Polymer materials: It can be used to prepare polymers, resins, and coatings.3. Pesticides: It is also used as an ingredient in pesticides to prevent or control pests and weeds on crops.Phenethyl isothiocyanate is generally prepared through chemical synthesis, commonly using the following methods:1. Reaction: Esterification of phenyl-2-ethanol with isothiocyanate produces phenethyl isothiocyanate.2. Thiocyanation reaction: Phenethyl alcohol reacts with sodium thiocyanate to form the corresponding nitrile, which is then hydrolyzed with acid to produce phenethyl isothiocyanate.Safety information for phenethyl isothiocyanate is as follows:1. Toxicity: Phenethyl isothiocyanate is moderately toxic to humans. Exposure or inhalation of large amounts may cause irritation and harm the respiratory and digestive systems.2. Flammability: It is a flammable liquid and may ignite or explode when exposed to open flames or high temperatures.3. Storage and handling: It should be stored in a cool, well-ventilated place, away from fire sources and oxidizers. Protective equipment should be worn during handling to avoid direct contact with skin and eyes, and inhalation of its vapors should be avoided.