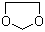1,3-Dioxolane(CAS#646-06-0)
| Hazard Symbols | F – Flammable |
| Risk Codes | 11 – Highly Flammable |
| Safety Description | 16 – Keep away from sources of ignition. |
| UN IDs | UN 1166 3/PG 2 |
| WGK Germany | 1 |
| RTECS | JH6760000 |
| TSCA | Yes |
| HS Code | 29329970 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: 3000 mg/kg LD50 dermal Rabbit 9074 mg/kg |
Introduction
Dioxide pentacycline (also known as pentacyclodioxide, cyclopentadiene glycol) is a chemical compound. The following is an introduction to the properties, uses, preparation methods and safety information of dioxide pentacyc:Properties: Dioxide is a colorless to light yellow liquid with a specific odor. It is insoluble in water, but soluble in alcohols, ethers, and certain organic solvents. It is less dense and easily volatilizes at room temperature.Usage: Dioxide is commonly used as a solvent in the manufacturing and processing of various chemical products such as coatings, inks, and resins. It can also be used in the study of synthetic organic compounds, such as environmental analysis, fine chemical synthesis, etc.Preparation method: The preparation of dioxentacycles is mainly obtained by the oxidation reaction of glutaric acid or pentanol. One of the commonly used methods is to oxidize glutaric acid or pentanol to dioxidation using oxygen or oxidants.Safety Information: Dioxide is a flammable liquid that poses a risk of combustion and explosion, and should be kept away from open flames and high temperatures. When using or storing, it is essential to maintain good ventilation and avoid inhaling its vapors. Exposure to dioxide may cause skin and eye irritation, and necessary safety measures should be taken, such as wearing protective gloves, goggles, and protective clothing. If you ingest or inhale it, you should seek medical help immediately. When handling or disposing of waste, follow the guidance of local laws and regulations.