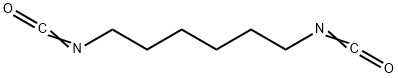
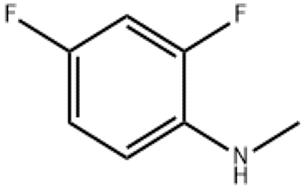
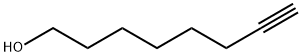
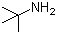Dimetol (CAS#543-49-7)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R21 – Harmful in contact with skin R36 – Irritating to the eyes |
| Safety Description | S36/37 – Wear suitable protective clothing and gloves. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| UN IDs | UN 1987 3/PG 3 |
| WGK Germany | 3 |
| RTECS | MJ2975000 |
| TSCA | Yes |
| HS Code | 29051900 |
| Hazard Class | 3 |
| Packing Group | III |
| Toxicity | LD50 orally in Rabbit: 2580 mg/kg LD50 dermal Rabbit 1460 mg/kg |
Introduction
2-Heptanol is a chemical substance also known as 1-amyl alcohol. The following is an introduction to the properties, uses, preparation methods and safety information of 2-heptanol:Quality:- The appearance of a colorless liquid with a specific alcoholic odor.– Soluble in water, ethers and alcohols, miscible with many organic solvents.Use:- 2-Heptanol is widely used in chemicals, pharmaceuticals, and fragrances.- In industry, it can be used in the production of solvents, lubricants, and softeners.- It can also be used to prepare chemical products such as esters, plastics, and synthetic resins.- In the fragrance industry, it can be used to create perfumes and fragrances with fruity aromas.Preparation method:- 2-Heptanol is generally synthesized by catalyzed hydrogenation reaction through sodium hydroformate.- This synthesis process usually uses heptene as a raw material, and the product is obtained by hydrogenation reaction of hydrogen under high pressure and under the action of a catalyst.Safety Information:- Avoid direct contact with the skin and eyes to avoid irritation and damage.- Good ventilation should be provided during use to avoid inhaling its vapors.- When used and stored, it is necessary to keep it away from fire sources and other flammable substances.- Seek immediate medical attention in case of accidental ingestion or heavy exposure.