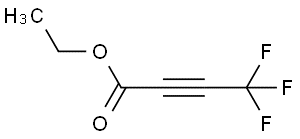
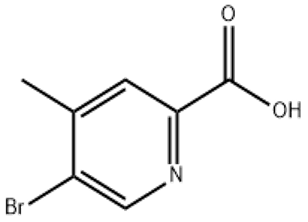
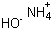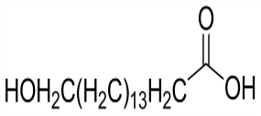Ammonium hydroxide(CAS#1336-21-6)
| Risk Codes | R34 – Causes burns R50 – Very Toxic to aquatic organisms R22 – Harmful if swallowed |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 2672 8/PG 3 |
| WGK Germany | 2 |
| RTECS | BQ9625000 |
| FLUKA BRAND F CODES | 34 |
| TSCA | Yes |
| HS Code | 28142000 |
| Hazard Class | 8 |
| Packing Group | III |
| Toxicity | LD50 oral (rat) 350 mg/kg PEL (OSHA) 35 ppm (27 mg/m3) TLV-TWA (ACGIH) 25 ppm (17 mg/m3) STEL (ACGIH) 35 ppm (27 mg/m3) |
Introduction
Ammonia), also known as ammonia solution, is an inorganic compound solution. The following is an introduction to the properties, uses, preparation methods and safety information of ammonia:Quality:- Ammonia is a colorless liquid with a specific ammonia smell.- It is an alkaline solution that produces hydroxide ions (OH-) in water, which have the properties of an alkali.- Ammonia has good solubility and can dissolve many substances such as acids, salts, etc.- Ammonia is reductive and can react with some metal ions.Use:- Ammonia is widely used in various fields. In industry, ammonia is commonly used in the manufacture of fertilizers, detergents, cleaning agents, glass manufacturing, metal surface treatment, etc. In laboratories, ammonia is often used to conduct chemical experiments and prepare some reagents.Preparation method:- There are several methods for preparing ammonia, including two main methods:2. Ammonia salt hydrolysis: ammonia salts (such as ammonia hydrochloric acid, ammonia nitrate, etc.) are reacted with water to produce ammonia.Safety Information:- Ammonia has a pungent odor and high concentrations of gas are toxic. When exposed to ammonia, keep away from fire and other flammable materials.- Ammonia is an alkaline substance that can cause burns and irritation when it comes into contact with the skin. Personal protective equipment such as protective gloves and glasses should be worn when using it.- When storing and using ammonia, it should be kept away from acids, oxidants and other substances to avoid dangerous reactions.- If you accidentally ingest or inhale too much ammonia, you should seek immediate medical attention. Immediately after contact with ammonia, rinse the affected area with plenty of water and seek medical attention.