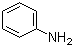
Aniline(CAS#62-53-3)
| Risk Codes | R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R40 – Limited evidence of a carcinogenic effect R41 – Risk of serious damage to eyes R43 – May cause sensitization by skin contact R48/23/24/25 - R50 – Very Toxic to aquatic organisms R68 – Possible risk of irreversible effects R48/20/21/22 - R39/23/24/25 - R11 – Highly Flammable |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S27 – Take off immediately all contaminated clothing. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S46 – If swallowed, seek medical advice immediately and show this container or label. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S63 - S36/37 – Wear suitable protective clothing and gloves. S16 – Keep away from sources of ignition. |
| UN IDs | UN 1547 6.1/PG 2 |
| WGK Germany | 2 |
| RTECS | BW6650000 |
| FLUKA BRAND F CODES | 8-9 |
| TSCA | Yes |
| HS Code | 2921 41 00 |
| Hazard Class | 6.1 |
| Packing Group | II |
| Toxicity | LD50 orally in rats: 0.44 g/kg (Jacobson) |
Introduction
Aniline is an organic compound, and the following is an introduction to the properties, uses, preparation methods and safety information of aniline:Quality:- Aniline is a colorless liquid with a strong pungent odor at room temperature.- Soluble in water and organic solvents, with strong alkalinity.- Aniline is a strong reducing agent that reacts violently with oxidants such as oxygen and chlorine.- Prone to oxidation and gradually darken when exposed to air.Use:- It is an important raw material for the production of synthetic fibers, rubber antioxidants, etc.Preparation method:- Aniline is usually prepared by the reduction of phenyl nitrate or the epoxidation of aromatic amines.- Phenylnitric acid reduction is the most common method of preparing aniline, which is obtained by reacting phenyl nitric acid with a reducing agent such as sulfite.Safety Information:- Aniline is highly irritating and can cause damage to the skin, eyes, and respiratory system, so contact should be avoided.- Long-term exposure to aniline can cause damage to the central nervous system and hematopoietic system.- Aniline is a carcinogen that may be associated with the development of leukemia and other malignant tumors.- Proper safety measures must be taken when handling aniline, such as wearing protective gloves, goggles, and protective clothing, to ensure safe handling and good ventilation.