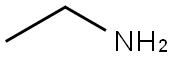
Ethylamine(CAS#75-04-7)
| Risk Codes | R12 – Extremely Flammable R36/37 – Irritating to eyes and respiratory system. R19 – May form explosive peroxides R39/23/24/25 - R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R11 – Highly Flammable R37 – Irritating to the respiratory system R35 – Causes severe burns R24 – Toxic in contact with skin R22 – Harmful if swallowed R10 – Flammable R40 – Limited evidence of a carcinogenic effect |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S29 – Do not empty into drains. S16 – Keep away from sources of ignition. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37 – Wear suitable protective clothing and gloves. S7 – Keep container tightly closed. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| UN IDs | UN 2733 3/PG 1 |
| WGK Germany | 1 |
| RTECS | KH2100000 |
| FLUKA BRAND F CODES | 4.5-31 |
| TSCA | Yes |
| HS Code | 29211990 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in rats: 0.40 g/kg (Smyth) |
Introduction
Ethylamine is a colorless gas with a pungent odor. Ethylamine is a primary amine, which is basic and can react with acids in neutralization reactions.Ethylamine has a wide range of applications. It can also be used in the synthesis of dyes, resins, amine compounds, and more. In chemical research, ethylamine is widely used in organic synthesis reactions.There are mainly two methods for preparing ethylamine. One is by reacting ammonia with ethanol to produce ethylamine and water. The other is by catalytic hydrogenation of ammonia with ethylene. These methods are widely used in industrial production.Ethylamine has a pungent odor, and excessive inhalation can cause irritation to the eyes, respiratory tract, and skin. When storing and using ethylamine, care should be taken to prevent contact with oxidizing agents such as oxygen or chlorine to avoid fire or explosion. Ethylamine can absorb moisture from the air and form flammable vapors, so it should be stored in a dry, well-ventilated place.