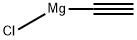ETHYNYLMAGNESIUM CHLORIDE(CAS#65032-27-1)
| Risk Codes | R11 – Highly Flammable R14 – Reacts violently with water R19 – May form explosive peroxides R22 – Harmful if swallowed R34 – Causes burns R40 – Limited evidence of a carcinogenic effect R36/37 – Irritating to eyes and respiratory system. R63 – Possible risk of harm to the unborn child R48/20 - R36/37/38 – Irritating to eyes, respiratory system and skin. R14/15 - |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37 – Wear suitable protective clothing and gloves. S43B - S33 – Take precautionary measures against static discharges. S29 – Do not empty into drains. S6A - |
| UN IDs | UN 3399 4.3/PG 2 |
| WGK Germany | 1 |
| HS Code | 29319090 |
Introduction
ethynylmagnesium chloride is an organometallic compound with the chemical formula C2H2MgCl. It is a colorless solid, soluble in ether and alkane solvents.
ethynylmagnesium chloride are often used in organic synthesis reactions, such as Grignard reaction and Soxhaft synthesis reaction. It can be used as a carbon-seeking electron reagent and react with other compounds to generate carbon-carbon or carbon-functional group bonds.
A common method of preparing ethynylmagnesium chloride is by reacting sodium acetylide with magnesium chloride in an anhydrous environment. First, sodium acetylide is reacted with an anhydrous solvent (e. g., dry ether) to form sodium acetylide. Ethynyl sodium is then reacted with magnesium chloride to produce ethynylmagnesium chloride.
Regarding safety information, ethynylmagnesium chloride is a deliquescent substance and should be stored in a dry environment. It gradually decomposes in air and water, producing toxic gases. Therefore, appropriate protective equipment such as gloves and goggles should be worn when used in a work environment. In addition, ethynylmagnesium chloride should be stored in sealed containers, away from fire and oxidizing agents.