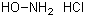Hydroxylamine hydrochloride(CAS#5470-11-1)
| Risk Codes | R22 – Harmful if swallowed R36/38 – Irritating to eyes and skin. R43 – May cause sensitization by skin contact R48/22 – Harmful danger of serious damage to health by prolonged exposure if swallowed. R50 – Very Toxic to aquatic organisms R40 – Limited evidence of a carcinogenic effect R21/22 – Harmful in contact with skin and if swallowed. R2 – Risk of explosion by shock, friction, fire or other sources of ignition |
| Safety Description | S22 – Do not breathe dust. S24 – Avoid contact with skin. S37 – Wear suitable gloves. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S36/37 – Wear suitable protective clothing and gloves. |
| UN IDs | UN 2923 8/PG 2 |
| WGK Germany | 3 |
| RTECS | NC3675000 |
| FLUKA BRAND F CODES | 21 |
| TSCA | Yes |
| HS Code | 28251000 |
| Hazard Class | 8 |
| Packing Group | III |
| Toxicity | LD50 orally in mice: 408 mg/kg (Riemann) |
Introduction
The following is a detailed introduction to the properties, uses, preparation methods and safety information of hydroxylamine hydrochloride:Quality:1. Hydroxylamine hydrochloride has the property of being miscible with water and can be soluble in organic solvents such as alcohols and ethers.2. It is reductive and reacts violently when reacting with oxidizing agents such as acidic potassium permanganate.3. Hydroxyamine hydrochloride is highly corrosive and can cause irritation and damage in contact with the skin and eyes.Use:1. Hydroxylamine hydrochloride is mainly used as a reducing agent and reagent in organic synthesis reactions. It is commonly used to reduce compounds such as nitrites, aldehydes, and ketones.2. Hydroxylamine hydrochloride can also be used to prepare other compounds, such as hydroxylamine hydrochloride, used as a rubber additive, antioxidant, etc.Preparation method:Hydroxylamine hydrochloride can be prepared by a variety of methods, two of which are commonly used:1. Ammonia reacts with hydrogen peroxide to obtain hydroxamine, and then reacts with hydrochloric acid to obtain hydroxylamine hydrochloride.2. Add ammonium sulfate and ammonia to alcohol, and heat it to produce ammonia, which reacts with hydrogen peroxide to form hydroxylamine, and then reacts with hydrochloric acid to form hydroxylamine hydrochloride.Safety Information:1. Hydroxylamine hydrochloride is corrosive and irritating, so you should pay attention to wearing protective glasses, gloves, clothing and other protective equipment when using it.2. Avoid contact with skin or eyes, and if accidentally touched, rinse immediately with plenty of water for at least 15 minutes, and seek medical attention if necessary.3. During use and storage, it should be kept away from fire and humid environments.4. Hydroxylamine hydrochloride is a dangerous product and should be stored in a sealed container away from other substances to prevent reaction with moisture in the air.