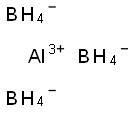
Water(CAS#7732-18-5)
| Safety Description | 26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| RTECS | ZC0110000 |
| FLUKA BRAND F CODES | 34 |
| HS Code | 28530010 |
Introduction
Water is a colorless and odorless liquid. It has many unique properties. Water is highly soluble and can dissolve many substances, making it known as a “universal solvent.” Secondly, water has a high specific heat capacity and latent heat of evaporation, which can absorb and release a large amount of heat and regulate temperature. Water also has surface tension and viscosity, which can form water droplets and water columns.Water is widely used in various fields. It is also essential for maintaining good health for body cleansing, hygiene, and rinsing. Water is used for agricultural irrigation, industrial production, and energy generation. In chemical laboratories, water is a commonly used reaction solvent.There are two main methods of water preparation: natural acquisition and artificial production. Naturally available water is mainly through natural sources such as groundwater, lakes, rivers and oceans. Artificial water manufacturing includes methods such as distillation, filtration, and ionization.In terms of safety, water is generally safe for humans and the environment. In some industrial processes, water may react with chemicals to produce harmful substances.