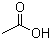
Acetic acid glacial(CAS#64-19-7)
| Risk Codes | R34 – Causes burns R42 – May cause sensitization by inhalation R35 – Causes severe burns R10 – Flammable R36/38 – Irritating to eyes and skin. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S23 – Do not breathe vapour. S24/25 – Avoid contact with skin and eyes. |
| UN IDs | UN 1792 8/PG 2 |
| WGK Germany | 3 |
| RTECS | NN1650000 |
| FLUKA BRAND F CODES | 1-8-10 |
| TSCA | Yes |
| HS Code | 29152100 |
| Hazard Class | 8 |
| Packing Group | II |
| Toxicity | LD50 in rats (g/kg): 3.53 orally (Smyth) |
Introduction
Acetic acid. The following is an introduction to the properties, uses, preparation methods and safety information of acetic acid:Quality:- Acetic acid has a pungent odor, tastes sour, and is soluble in water and most organic solvents.- It is a weak acid that reacts neutrally with alkalis to form acetate.- Acetic acid can chemically react with many substances, such as alcohols, aldehydes, nitric acid, etc.Use:- It is also an important substance in the manufacture of certain chemical raw materials such as plastics, fibers, rubber, etc.- In laboratories, acetic acid is commonly used in chemical analysis and synthesis reactions.Preparation method:- Acetic acid is mainly obtained by fermentation in industrial production. First, yeast is used to catalyze the oxidation of ethanol to acetaldehyde, and then acetaldehyde is further oxidized to acetic acid under the action of acetic acid bacteria.- Acetic acid can be obtained in the laboratory by using oxidants for the oxidation of ethanol.Safety Information:- Acetic acid is a corrosive substance that should be handled carefully and avoided in contact with skin and eyes.- Proper ventilation should be taken when using acetic acid to avoid inhaling its vapors.Please follow relevant safety operating procedures when using or handling acetic acid, and pay attention to the safety warning information on the specific product label.