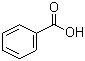
Benzoic acid(CAS#65-85-0)
| Risk Codes | R22 – Harmful if swallowed R36 – Irritating to the eyes R42/43 – May cause sensitization by inhalation and skin contact. R36/37/38 – Irritating to eyes, respiratory system and skin. R40 – Limited evidence of a carcinogenic effect R63 – Possible risk of harm to the unborn child R43 – May cause sensitization by skin contact R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R45 – May cause cancer R41 – Risk of serious damage to eyes R37/38 – Irritating to respiratory system and skin. R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R48/23 - R38 – Irritating to the skin R67 – Vapors may cause drowsiness and dizziness R37 – Irritating to the respiratory system |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S37/39 – Wear suitable gloves and eye/face protection S24 – Avoid contact with skin. S22 – Do not breathe dust. S36/37 – Wear suitable protective clothing and gloves. S24/25 – Avoid contact with skin and eyes. S23 – Do not breathe vapour. S53 – Avoid exposure – obtain special instructions before use. S36 – Wear suitable protective clothing. S63 - S39 – Wear eye / face protection. |
| UN IDs | UN 3077 9/PG 3 |
| WGK Germany | 1 |
| RTECS | DG0875000 |
| FLUKA BRAND F CODES | 21 |
| TSCA | Yes |
| HS Code | 2916 31 00 |
| Hazard Note | Harmful |
| Toxicity | LD50 orally in Rabbit: 1700 mg/kg LD50 dermal Rabbit > 5000 mg/kg |
Introduction
Benzoic Acid. The following is an introduction to the properties, uses, preparation, and safety information of benzoic acid:Properties:- Appearance: Benzoic acid is a white crystalline solid, soluble in water and organic solvents.- Chemical properties: Benzoic acid is acidic and can react with bases to form the corresponding salts.- Chemical structure: The benzoic acid molecule consists of a benzene ring with a carboxyl group (-COOH) attached to it.Uses:- Chemical synthesis: Benzoic acid is an important raw material for the synthesis of flavors, dyes, flexible polyurethane, and fluorescent substances.Preparation:- From benzene: Benzoic acid can be obtained by oxidizing benzene with oxygen in the presence of a catalyst.- From toluene: Benzoic acid can be obtained by oxidizing toluene with oxygen in the presence of a catalyst.Safety Information:- Benzoic acid is relatively safe for humans at room temperature, but the following precautions should still be observed:- Contact: Avoid direct contact with the skin and eyes. In case of contact, rinse immediately with plenty of water and seek medical attention promptly.- Inhalation: Avoid prolonged inhalation of benzoic acid vapors. Operate in well-ventilated areas.- Ingestion: Benzoic acid is somewhat toxic and must not be ingested.- Storage: Store benzoic acid away from fire and oxidizing agents to prevent combustion.