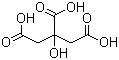
Citric acid(CAS#77-92-9)
| Risk Codes | R41 – Risk of serious damage to eyes R36/37/38 – Irritating to eyes, respiratory system and skin. R36/38 – Irritating to eyes and skin. R37/38 – Irritating to respiratory system and skin. R34 – Causes burns R36 – Irritating to the eyes R35 – Causes severe burns R61 – May cause harm to the unborn child R60 – May impair fertility |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S39 – Wear eye / face protection. S37/39 – Wear suitable gloves and eye/face protection S24/25 – Avoid contact with skin and eyes. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36 – Wear suitable protective clothing. S53 – Avoid exposure – obtain special instructions before use. |
| UN IDs | UN 1789 8/PG 3 |
| WGK Germany | 1 |
| RTECS | GE7350000 |
| FLUKA BRAND F CODES | 9 |
| TSCA | Yes |
| HS Code | 2918 14 00 |
| Toxicity | LD50 in mice, rats (mmol/kg): 5.0, 4.6 i.p. (Gruber, Halbeisen) |
Introduction
Citric acid is an organic acid. It is a colorless and odorless crystalline powder that dissolves in water and ethanol.The main uses of citric acid are as follows:Detergents: They have cleansing and dissolving effects and are commonly used in products such as detergents, hand sanitizers, and dish soaps.There are two main common methods for making citric acid:By fermentation: Using the fermentation of citric acid bacteria, substances containing sucrose or starch (such as molasses, malt, etc.) are fermented, and finally citric acid is extracted and crystallized.Through chemical synthesis: Citric acid is synthesized by chemical reactions, and commonly used raw materials include methanol, ethylene and oxidants.Citric acid is generally safe in normal doses, but excessive intake may cause stomach pain, vomiting, diarrhea and other digestive discomforts.When using citric acid, the correct method and dosage should be followed and must not be abused.Citric acid is a non-flammable substance, but it may decompose and release toxic gases at high temperatures, so contact with strong oxidants should be avoided.