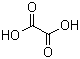
Oxalic acid(CAS#144-62-7)
Oxalic acid(CAS#144-62-7)
quality
Anhydrous oxalic acid is a colorless, odorless solid with deliquescence, and is available in two crystal forms: diamond-shaped and monoclinic. At room temperature, rhombus-shaped oxalate crystals are thermodynamically stable, while monoclinic oxalic acid crystals are thermodynamic metastability. Anhydrous oxalic acid melting point 189.5°C (decomposition). Partial decomposition begins at 157°C I When the heating is accelerated, all oxalic acid decomposes to form formic acid, carbon monoxide and water. It is soluble in water and alcohol, slightly soluble in ether, and insoluble in benzene, chloroform and petroleum ether. When exposed to high heat, open flame or contact with oxidizing agents, there is a risk of combustion, and toxic gases are produced by heating and decomposing.
Preparation method
The furnace gas is refined and pressurized, and the sodium hydroxide solution is in countercurrent contact at a certain temperature to produce sodium formate. Sodium formate crystallization is obtained by evaporation and concentration and centrifugation separation. Sodium formate is converted into sodium oxalate in a calciner, washed and filtered to remove sodium carbonate, and then calcium oxalate generated by slaked lime reaction is added to filter out and decomposed with sulfuric acid.
Calcium sulfate is filtered out, and the resulting oxalic acid crystals are refined by recrystallization method, or by using a mixture containing 30%~40% sulfuric acid and 20%~25% nitric acid and vanadium pentoxide catalyst, and oxalic acid is prepared by oxidation of ethylene glycol at a certain temperature and pressure, or glucose, sucrose, starch, dextrin and syrup and other carbohydrates are used as raw materials, and oxalic acid is prepared in the presence of vanadium catalyst by oxidation of nitrate monosulfuric acid. The crude product is resolved in mother liquor and hot water, and is prepared by degreasing, separation, filtration and recrystallization.
use
Mordants in the printing and dyeing industry, mainly used as reducing agents and bleaching agents, are used to refine rare metals, synthesize oxalates, oxalates and oxalammonium, etc.