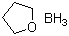Borane-tetrahydrofuran complex(CAS#14044-65-6)
| Risk Codes | R14/15 - R19 – May form explosive peroxides R22 – Harmful if swallowed R36/37/38 – Irritating to eyes, respiratory system and skin. R41 – Risk of serious damage to eyes R37/38 – Irritating to respiratory system and skin. R11 – Highly Flammable R67 – Vapors may cause drowsiness and dizziness R66 – Repeated exposure may cause skin dryness or cracking R40 – Limited evidence of a carcinogenic effect R36/37 – Irritating to eyes and respiratory system. |
| Safety Description | S16 – Keep away from sources of ignition. S33 – Take precautionary measures against static discharges. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S7/9 - S7/8 - S43 – In case of fire use … (there follows the type of fire-fighting equipment to be used.) S37/39 – Wear suitable gloves and eye/face protection S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S29 – Do not empty into drains. S36/37 – Wear suitable protective clothing and gloves. |
| UN IDs | UN 3399 4.3/PG 1 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 1-10-13-34 |
| TSCA | Yes |
| HS Code | 29321900 |
| Hazard Class | 4.3 |
| Packing Group | I |
Introduction
Tetrahydrofuran borane (THF-B2H6) is an organoboron compound with the following properties:Solubility: It can dissolve many organic substances, including alcohols, ketones, ethers, etc.Instability: Tetrahydrofuran borane is easy to decompose in air and can also decompose at high temperatures or light conditions.The main uses of tetrahydrofuran borane are as follows:Organic Synthesis: It is a commonly used organic synthesis reagent that can be used for the reduction reactions of alcohols, ketones, aldehydes, and other compounds.Metal-organic chemistry: It can be used as a reducing agent and additive for many metals.Catalyst: Tetrahydrofuran borane can play a role in organic synthesis as an important catalyst.Electronic materials: It has high active carrier mobility and good electron transport characteristics, and can be used in the preparation of electronic devices such as organic batteries.The preparation method of tetrahydrofuran borane mainly includes:Through the reaction of boron hydride and tetrahydrofuran, the reaction conditions are carried out at room temperature.By the reaction of trichloroboron and tetrahydrofuran, heating and stirring are required.Avoid contact with skin and eyes: Tetrahydrofuranoborane is irritating to the skin and eyes and should avoid direct contact.Avoid inhalation and ingestion: During use, care should be taken to prevent inhalation of gas or accidental ingestion. In case of inhalation or ingestion, transfer to fresh air immediately and seek medical attention as soon as possible.Storage conditions: Tetrahydrofuran borane should be stored in a cool, dry and well-ventilated place, away from fire sources and oxidants. Avoid direct sunlight and heat.